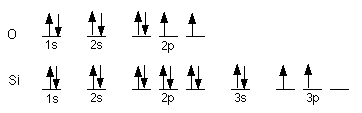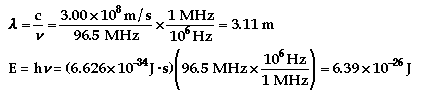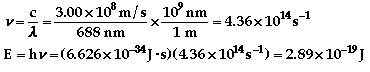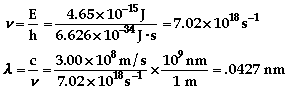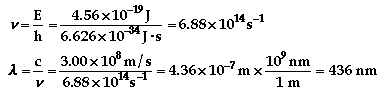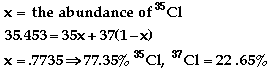Answer Key for the Practice
Questions for Atomic Theory
- a)
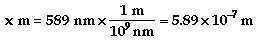
b) 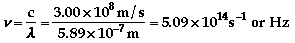
c)
This is in the visible spectrum.
d)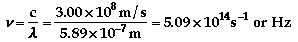
- Quantum theory only allows for
discrete energies to be emitted, not energies at all
wavelengths. The discrete energies come from the spacing
of the energy levels in which the electrons in potassium
move around. See the atomic
theory additional
information for more of an explanation.
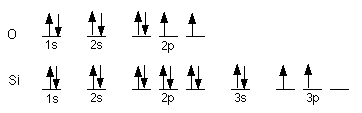
There are 2 unpaired electrons
in each. - a) 1s22s22p1
- correct b) 1s22s13s1
excited c) 1s22s22p63s22d2
incorrect d) 1s22s42p2
incorrect e) 1s12s1excited
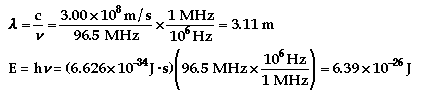
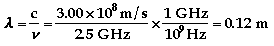
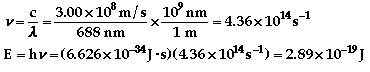
The color of light associated with
688 nm is red. 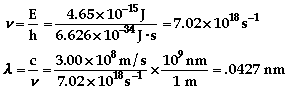
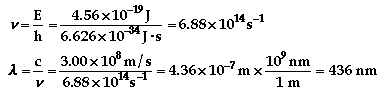
The color of light associated with
436 nm is violet.
Answer Key for
the Atom and Atomic Theory Review Questions
- D = m/V = 3.44
g/2.77 mL = 1.24 g/mL
- V = m/D = 50.0
g/1.19 x 10-3 g/cm3 = 4.2 x 104
cm3.
- For the four
main ideas of Dalton's atomic theory, see your book, p.
84.
- Electrons were
discovered by J.J. Thomson using the cathode ray tube.
See pp.86-88 for the complete description.
- Rutherford
shot alpha particles through thin gold foil. He expected
all of the particles to go straight through. Most did,
but some deflected. This lead him to two conclusions:
first, that atoms are mostly empty space, and secondly,
that there is a positively charged, dense nucleus present.
See your book for more of a description.
- a) Ba: p+=56,
e-= 56, no= 81 b) Cu: p+=
29, e-= 29, no= 35 c) F:
p+= 9, e-= 9, no= 10
- An isotope of
an element contains the same number of protons and
electrons but different number of neutrons.
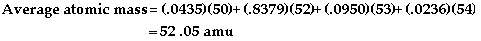
- J.J. Thomson's
model was the "plum pudding" model - a
positively charged blob with negative electrons stuck
throughout it. Rutherford stated that there was a small,
dense, positively charged nucleus with electrons outside
the nucleus. Bohr also had a positive nucleus, but stated
that the electrons were in definite orbits, or energy
levels, around the nucleus. The quantum mechanical model
also gives a positive nucleus, but states that the
electrons are in not in a definite orbit, but rather in a
probability region, or electron cloud.
- An atomic
orbital is the region of space around a nucleus where an
electron will most likely be (probability region).
- An s orbital
has a spherical shape. It holds 2 electrons. A p orbital
has a dumbbell shape. It also holds 2 electrons, however
there are three p orbitals, one oriented along the x-axis,
another along the y-axis, and the third along the z-axis.
- See your text.
- a) S 1s22s22p63s23p4,
[Ne] 3s23p4
b) Rb 1s22s22p63s23p64s23d104p65s1,
[Kr] 5s1
c) Au 1s22s22p63s23p64s23d104p65s24d105p66s24f145d9,
though this is an exception and actually ends in 6s14f145d10,
[Xe] 6s24f145d9 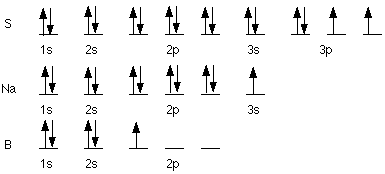
- lowest
wavelength: violet, indigo, blue, green, yellow, orange,
red: highest wavelength
- The electron
in the hydrogen atom can be excited to various energy
levels by absorbing energy. As it relaxes back to a lower
state, it releases energy. By relaxing from different
levels, different amounts of energy will be released,
which produce a different color of light. See the atomic
theory additional
information for more of an explanation.
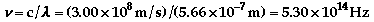
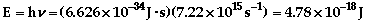
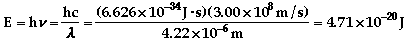
- The ground state is the lowest
energy level for an electron to occupy. An excited state
is when an electron is above the lowest energy level
available.
- Heisenberg's uncertainty principle
states that both the position and velocity of an electron
cannot be known at the same time.
- de Broglie hypothesized that all
matter can exhibit wavelike properties. He derived an
equation to describe the wavelength of a particle
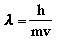 where h is
Planck's constant, m is the mass of the particle, and v
is its velocity.
where h is
Planck's constant, m is the mass of the particle, and v
is its velocity.
- The photoelectric effect is where a
photon (packet of light) can eject an electron from a
metal. The photon must be at least at the threshold
energy for an electron to be ejected. For a complete
description, see your book.
Notes:
E = energy (Joules)
f = frequency (hertz)
c = speed of light = 3.00
x 108 m/s
h = Planck's constant =
6.626 x 10-34 J/s
an upside down Y is
lamda = wavelength (m)
m = mass
v = velocity
Return to the Atoms and Atomic Spectra
Return to
Donkistry Worksheets
![]()
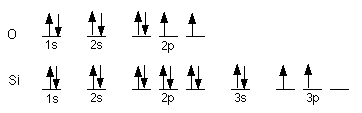
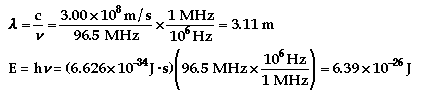
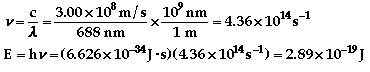
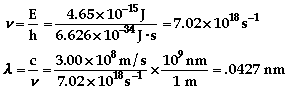
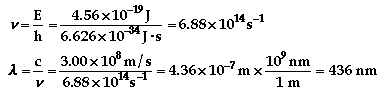
![]()