5Br-(aq) + BrO3-(aq) + 6H+(aq) --> 3Br2(aq) + 3H2O(l)
If the rate of appearance of Br2 at a particular moment during the reaction is 0.025 M s-1, what is the rate of disappearance (in M s-1) of Br- at that moment?
(CH3)3COH(l) + HCl(aq) --> (CH3)3CCl(l) + H2O(l)
The experimentally determined rate law for this reaction indicates that the reaction is first-order in (CH3)3COH and that the reaction is first-order overall. Which of the following would produce an increase in the rate of this reaction?
- increasing the concentration of (CH3)3COH
- increasing the concentration of HCl
- decreasing the concentration of HCl
- decreasing the concentration of (CH3)3CCl
- It is impossible to tell.
- temperature
- activation energy
- presence of a catalyst
- concentrations of reactants
- All of the above influence the rate.

Which of the following diagrams represents a snapshot of a very small portion of this system at t = 3 min?

H2(g) + 2 NO(g) --> N2O(g) + H2O(g)
A study of initial concentration (ha, ha!) versus initial rate at a certain temperature yields the following data for this reaction (ha, ha!):
| [H2], M | [NO], M | initial rate, M s-1 |
|---|---|---|
| 0.1000 | 0.5000 | 2.560 x 10-6 |
| 0.2000 | 0.3000 | 1.843 x 10-6 |
| 0.1000 | 0.3000 | 9.216 x 10-7 |
| 0.2000 | 0.6000 | 7.373 x 10-6 |
Which of the following is the correct rate law for this reaction (ha, ha!)?
- Rate = k[H2][NO]2
- Rate = k[H2][NO]
- Rate = k[NO]2
- Rate = k[H2]2
- Rate = k
A + B --> Products Rate = k[A][B]
What are the relative rates of this reaction in the vessels shown below. Note: each vessel has the same volume.

- III > I = IV > II
- I > III > II > IV
- IV > II > III > I
- II > IV > I > III
- III > I > IV > II
[Cr(NH3)5Cl]2+(aq) + OH-(aq) --> [Cr(NH3)5(OH)]2+(aq) + Cl-(aq)
The following data were obtained for this reaction at 25oC,
| time, min | [Cr(NH3)5Cl]2+, M |
|---|---|
| 0 | 1.00 |
| 6 | 0.657 |
| 12 | 0.432 |
| 18 | 0.284 |
| 24 | 0.186 |
| 30 | 0.122 |
| 36 | 0.0805 |
The order of the reaction with respect to the [Cr(NH3)5Cl]2+ ion is:
- zero order
- first order
- second order
- third order
- fourth order
- k versus T
- k versus (1/T)
- ln k versus (1/T)
- ln k versus T
- ln k versus Ea
- The Spectronic 20 becomes unstable towards the end of the reaction.
- Towards the end of the reaction, the temperature of the solution is significantly different than the initial temperature of the solution.
- Towards the end of the reaction, the concentrations of the reactants are so high that it is difficult to measure them accurately.
- Towards the end of the reaction, the concentrations of the products are sufficiently high that the reverse reaction competes with the forward reaction.
- None of these.
The next two questions are about this reaction:
2N2O5 (g) <==> 4NO2 (g) + O2 (g)
- rate = k [N2O5]2
- rate = [N2O5]2
- rate = k [N2O5]2 / [NO2]4 [O2]1
- rate = k [N2O5]x
- rate = [N2O5]x
- 0
- 1
- 2
- 3
- Cannot be determined.
The next two questions are about this reaction:
2NO(g) + Cl2(g) <==> 2NOCl(g)
rate = k[NO][Cl2].
What is the overall order of the reaction?
- 0
- 1
- 2
- 3
- Cannot be determined.
| (1) | NO(g) + Cl2(g) --> NOCl2(g) |
| (2) | NOCl2(g) + NO(g) --> 2NOCl(g) |
Based on the rate law given in the preceding problem, which step is the rate-limiting step?
- Step (1)
- Step (2)
- Both Steps (1) & (2)
- Either Steps (1) or (2)
I- (aq) + OCl- (aq) -> IO- (aq) + Cl- (aq)
and the following initial concentration and initial rate data for this reaction:
| [I-], M | [OCl-], M | initial rate, M s-1 |
|---|---|---|
| 0.1000 | 0.0500 | 3.05 x 10-4 |
| 0.2000 | 0.0500 | 6.10 x 10-4 |
| 0.3000 | 0.0100 | 1.83 x 10-4 |
| 0.3000 | 0.0200 | 3.66 x 10-4 |
Which of the following is the correct rate law for this reaction?
- Rate = k[I-]
- Rate = k[OCl-]
- Rate = k[I-]2
- Rate = k[I-][OCl-]
- Rate = k[I-]2[OCl-]
- The rate of the reaction is measured when the reaction is very close to equilibrium.
- The rate of the reaction is measured immediately after the reaction is started.
- The rate of the reaction is measured when the reaction is about one-half complete.
- The rate of the reaction is measured after five half-lives.
- The rate of an equilibrium reaction cannot be measured using this method.
- M s-1
- M-1 s-1
- M-2 s-1
- M-3 s-1
- M
- The existence of certain intermediates in a reaction mechanism can sometimes be proven because intermediates can sometimes be trapped and identified.
- Intermediates in a reaction mechanism cannot be isolated because they do not have finite lifetimes.
- Reaction mechanisms cannot have any more than one intermediate.
- Intermediates in a reaction mechanism appear in the overall, balanced equation for the reaction.
- None of the above statements is TRUE.
- A catalyst changes the potential energies of the reactants and products.
- A catalyst decreases the temperature of the reaction which leads to a faster rate.
- A catalyst lowers the activation energy for the reaction by providing a different reaction mechanism.
- A catalyst destroys some of the reactants, which lowers the concentration of the reactants.
- A catalyst raises the activation energy for the reaction which produces a faster rate.
- the change in free energy per second.
- the change in temperature per second.
- the number of collisions per second.
- the number of product molecules.
- none of the above.
2 NO (g) + 2 H2 (g) -> N2 (g) + 2 H2O (g)
If the mechanism for this reaction were,
| 2NO(g) + H2(g) -> N2(g) + H2O2(g) | slow |
| H2O2(g) + H2(g) -> 2H2O(g) | fast |
which of the following rate laws would we expect to obtain experimentally?
- Rate = k[H2O2][H2]
- Rate = k[NO]2[H2]
- Rate = k[NO]2[H2]2
- Rate = k[NO][H2]
- Rate = k[N2][H2O2]
5 Br- (aq) + BrO3- (aq) + 6 H+ (aq) -> 3 Br2 (aq) + 3 H2O (l)
If the rate of disappearance of Br- (aq) at a particular moment during the reaction is 3.5 x 10-4 M s-1, what is the rate of appearance of Br2 (aq) at that moment?
2 HI (g) -> H2 (g) + I2 (g)
and the following experimental data obtained at 555 K,
| [HI], M | rate, M s-1 |
|---|---|
| 0.0500 | 8.80 x 10-10 |
| 0.1000 | 3.52 x 10-9 |
| 0.1500 | 7.92 x 10-9 |
What is the order of the reaction with respect to HI (g)?
2 HI (g) -> H2 (g) + I2 (g)
The value of the rate constant, k, for this reaction was measured at several different temperatures and the data are shown below:
| temperature, K | k, M-1 s-1 |
|---|---|
| 555 | 6.23 x 10-7 |
| 575 | 2.42 x 10-6 |
| 645 | 1.44 x 10-4 |
| 700 | 2.01 x 10-3 |
Calculate the value of the activation energy (in kJ/mol) for this reaction.
- Rate laws for chemical reactions can be determined from the stoichiometry of the overall balanced equation.
- The rate of a chemical reaction that occurs in solution depends on the concentration, the temperature and the viscosity of the solvent.
- The "Method of Initial Rates" cannot be used for equilibrium reactions.
- Experimentally, transition states can sometimes be trapped or isolated whereas intermediates cannot be either trapped or isolated.
2 ClO2 (aq) + 2 OH- (aq) -> ClO3- (aq) + ClO2- (aq) + H2O (l)
Write an expression that describes the relationship between the rates of disappearance of ClO2 and OH- and the rates of appearance of ClO3-, ClO2- and H2O.
| k, s-1 | T, K |
|---|---|
| 3.94 x 10-4 | 384 |
| 1.17 x 10-3 | 397 |
| 5.26 x 10-2 | 447 |
| 4.63 x 10-1 | 481 |
NO (g) + O3 (g) -> NO2 (g) + O2 (g)
The experimentally determined rate law for this reaction is: Rate = k[O3]. Consider the following proposed mechanism for this reaction:
| Step 1: | O3 (g) -> O2 (g) + O (g) | fast |
| Step 2: | NO (g) + O (g) -> NO2 (g) | slow |
This proposed mechanism is NOT an acceptable possibility for this reaction. Briefly explain why this mechanism is not acceptable. ALSO, identify the rate-determining step and any intermediates in this proposed mechanism.
- The reaction proceeds faster as time increases.
- Intermediates are easier to detect.
- The reaction proceeds slower as time increases.
- Reverse reactions are avoided.
- The concentrations of the reactions hasn't changed much.
USE THE FOLLOWING DATA FOR THE NEXT THREE (3) QUESTIONS.
Nitric oxide gas reacts with chlorine gas according to the equation,
2 NO + Cl2 -> 2 NOCl
The following data were obtained for this reaction:
| initial [NO] | initial [Cl2] | initial rate |
|---|---|---|
| 0.50 M | 0.50 M | 1.19 mol/L hr |
| 1.00 | 0.50 | 4.79 |
| 1.00 | 1.00 | 9.59 |
| 1.50 | 1.50 | 32.27 |
- Rate = k[NO]
- Rate = k[NO][Cl2]1/2
- Rate = k[NO][Cl2]
- Rate = k[NO]2[Cl2]
- Rate = k[NO]2[Cl2]2
- 1
- 1.5
- 2
- 3
- 4
- 2.38
- 3.35
- 4.76
- 9.52
- 19.04
- Zero order.
- First order.
- Second order.
- A fractional order.
- Impossible to determine from this data.
2 NOBr(g) -> 2 NO(g) + Br2(g)
The reaction is known to be second order with k = 0.80 L/mol s. If you start with a concentration of 0.086 mol/L of NOBr, what will be its concentration after 22 seconds?
- 2.0 x 10-9 mol/L
- 5.4 x 10-2 mol/L
- 3.4 x 10-2 mol/L
- 8.7 x 10-1 mol/L
- 1.8 x 101 mol/L
- 0.000121
- 0.0300
- 0.197
- 3.51
- 33.4
- The temperature of the system.
- The orientation of the molecules at the moment of collision.
- The energy with which the collisions occur.
- The activation energy of the complex.
- All of these factors are important.
Rate = kobserved[NO2][O3]
Which of the following mechanisms is consistent with this experimental rate law?
(a) NO2 + NO2 <=> N2O4 (fast equilibrium)
N2O4 + O3 -> N2O5 + O2 (slow)
(b) NO2 + O3 -> NO5 (fast)
NO5 + NO5 -> N2O5 + 5/2 O2 (slow)
(c) NO2 + O3 -> NO3 + O2 (slow)
NO3 + NO2 -> N2O5 (fast)
(d) NO2 + NO2 -> N2O2 + O2 (slow)
N2O2 + O3 -> N2O5 (fast)
(e) None of these mechanisms are possible.
2 NOCl(g) -> 2 NO(g) + Cl2(g)
| temperature (K) | rate constant, k (L/mol s) |
|---|---|
| 400 | 6.6 x 10-4 |
| 500 | 2.9 x 10-1 |
| 600 | 1.63 x 101 |
- 1.00 x 102 J/K mol
- 1.23 x 103 J/K mol
- 1.05 x 105 J/K mol
- 1.34 x 106 J/K mol
- 1.22 x 108 J/K mol
- Kp for the reaction = 0.
- The activation energy is high.
- The standard free energy change for the reaction is > 0.
- These reactions are endothermic.
- The standard entropy change is < 0.
| H2O2 + I- -> H2O + IO- | (slow) |
| H2O + IO- -> H2O + 1/2 O2 + I- | (fast) |
The function of the I- in this reaction is to:
- Raise the
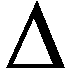 H of the products.
H of the products. - Lower the
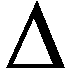 G between reactants and products.
G between reactants and products. - Increase the concentration of products.
- Lower the activation energy.
- Raise the
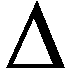 S of the reactants.
S of the reactants.
- Reaction 1: k = 2.9 x 10-7 M-1 s-1
- Reaction 2: k = 4.2 x 10-5 M-1 s-1
- Reaction 3: k = 7.8 x 102 M-1 s-1
- Reaction 4: k = 3.6 x 106 M-1 s-1
- There is insufficient information to answer this question.
2 H2O2 (aq) -> 2 H2O (l) + O2 (g)
If the average rate of disappearance of H2O2 over a certain time interval is 6.80 x 10-5 M s-1, what is the average rate of appearance of O2 during this same time interval?
- 4.62 x 10-9 M s-1
- 3.40 x 10-5 M s-1
- 6.80 x 10-5 M s-1
- 1.36 x 10-4 M s-1
- There is insufficient information to answer this question.
N2 (g) + 3 H2 (g) -> 2 NH3 (g)
Commercially, this reaction is performed at high temperature and in the presence of a heterogeneous catalyst (iron oxide) to increase the rate of the reaction. Which of the following "forms" of iron oxide would be the most effective for increasing the rate of the reaction?
- 1 g of iron oxide pellets (10 small spherical pellets)
- 1 g of iron oxide pellets (1 large spherical pellet)
- 1 g of iron oxide powder
- 1 g of iron oxide wire
- 1 g of iron oxide sitting on a table outside of the reaction vessel
C9H8O4 (aspirin) + H2O -> C2H4O2 (acetic acid) + C7H6O3
Consider the following initial concentration and initial rate data for this reaction:
| [Aspirin], M | [H2O], M | initial rate, M s-1 |
|---|---|---|
| 0.0100 | 0.0200 | 2.4 x 10-13 |
| 0.0100 | 0.0800 | 9.6 x 10-13 |
| 0.0300 | 0.0200 | 7.2 x 10-13 |
| 0.0200 | 0.0300 | 7.2 x 10-13 |
Which of the following is the correct rate law for this reaction?
- Rate = k[aspirin]
- Rate = k[aspirin][H2O]
- Rate = k[H2O]
- Rate = k[aspirin]2[H2O]
- Rate = k[aspirin]2[H2O]2
- spontaneous reactions
- reactions with large activation energies
- equilibrium reactions
- reactions carried out at low temperature
- all of the above
- 0.040 hours
- 5.70 hours
- 113 hours
- 226 hours
- 310 hours
2 HI (g) -> H2 (g) + I2 (g)
and the following data which were obtained at a temperature of 282oC:
| time, h | [HI] | 1/[HI] | ln [HI] |
|---|---|---|---|
| 0 | 0.900 | 1.111 | -0.105 |
| 200 | 0.733 | 1.364 | -0.311 |
| 400 | 0.618 | 1.618 | -0.481 |
| 600 | 0.534 | 1.873 | -0.627 |
Which of the following statements is TRUE?
- The decomposition of HI is a first-order process.
- The decomposition of HI is a zero-order process.
- A plot of ln [HI] versus time is linear with a slope of -k.
- A plot of 1/[HI] versus time is linear with a slope of +k.
- The half-life for this reaction is 180 hours.
A + B -> C + D + E
the value of the rate constant, k, was measured at several different temperatures and the data are shown below:
| temperature, oC | k, M-1 s-1 |
|---|---|
| 100 | 6.264 |
| 150 | 45.464 |
| 200 | 217.008 |
| 250 | 768.232 |
Calculate the value of the activation energy, Ea, for this reaction.
- 13.9 kJ
- 27.4 kJ
- 52.0 kJ
- 97.8 kJ
- 143 kJ
Cl2 (aq) + H2S (aq) -> S (s) + 2 HCl (aq)
The rate law for this reaction is found to be: Rate = k[Cl2][H2S]. Which of the following is an acceptable possibility for the mechanism of this reaction?
(a) Cl2 --> Cl+ + Cl- slow
Cl- + H2S --> HCl + HS- fast
Cl+ + HS- --> HCl + S fast
(b) Cl2 + H2S --> HCl + Cl+ + HS- slow
Cl+ + HS- --> HCl + S fast
(c) Cl2 --> Cl + Cl fast
Cl + H2S --> HCl + HS fast
HS + Cl --> HCl + S slow
(d) All of these mechanisms are acceptable possibilities.
(e) None of these mechanisms are acceptable possibilities.
H3O+ (aq) + OH- (aq) -> 2 H2O (l)
Which of the following statements is most likely TRUE?
- Increasing the temperature would have no effect on the rate of this reaction.
- The activation energy for this reaction must be very large.
- The rate of this reaction would be independent of the concentrations of H3O+ and OH-.
- The rate constant for this reaction would be different if the reaction were carried out in a more viscous solvent than water.
- None of the above.
Cl2 (g) + 3 F2 (g) -> 2 ClF3 (g)
Write an expression that describes the relationship between the rate of disappearance of Cl2, the rate of disappearance of F2 and the rate of appearance of ClF3.
2 MnO4- (aq) + 5 H2C2O4 (aq) + 6 H+ (aq) -> 2 Mn2+ (aq) + 10 CO2 (g) + 8 H2O (l)
Write the general form of the differential rate law for this reaction.
2 ClO2 (aq) + 2 OH- (aq) -> ClO3- (aq) + ClO2- (aq) + H2O (l)
and the following initial rate data:
| [ClO2], mol/L | [OH-], mol/L | initial rate, mol/L s |
|---|---|---|
| 0.0500 | 0.100 | 5.77 x 10-2 |
| 0.100 | 0.100 | 2.32 x 10-1 |
| 0.100 | 0.050 | 1.15 x 10-1 |
- Determine the order of each reactant and write the differential rate law for this reaction.
- Calculate the value of the rate constant,k, for this reaction. Be sure to include the appropriate units for the rate constant!
- What is the overall order for this reaction?
- Describe what would happen to the rate of this reaction if we tripled the concentration of ClO2 and doubled the concentration of OH-.
C2H4 (g) + H2 (g) -> C2H6 (g)
This is an example of heterogeneous catalysis which often involves gaseous reactants being adsorbed on the surface of a solid catalyst (i.e., PtO2). The rate of hydrogenation of ethylene on the surface of the PtO2 catalyst follows first-order kinetics for low concentrations of ethylene. However, as the concentration of ethylene is increased, the hydrogenation reaction becomes zero-order. Explain why the hydrogenation reaction in the presence of the heterogeneous catalyst PtO2 should be zero-order at high concentrations of ethylene.
H2 (g) + I2 (g) -> 2 HI (g)
The values for the rate constant, k, for this reaction are 2.45 x 10-4 L/mol s at 302oC and 0.950 L/mol s at 508oC.
- Calculate the value of the activation energy for this reaction.
- Calculate the value of the rate constant at 434oC.
NO2 (g) + CO (g) -> NO (g) + CO2 (g)
The experimentally determined rate law for this reaction is: Rate = k[NO2]2. A proposed mechanism for this reaction is shown below.
| Step 1: | NO2 (g) + NO2 (g) -> NO3 (g) + NO (g) | slow |
| Step 2: | NO3 (g) + CO (g) -> NO2 (g) + CO2 (g) | fast |
Determine whether this is a reasonable mechanism for this reaction and identify the rate-determining step and any intermediates in this proposed mechanism. Be sure to explain your reasoning.
2H2(g) + 2NO(g) --> 2H2O(g) + N2(g)
| experiment | [H2], M | [NO], M | initial rate, M s-1 |
|---|---|---|---|
| 1 | 0.010 | 0.025 | 2.4 x 10-6 |
| 2 | 0.0050 | 0.025 | 1.2 x 10-6 |
| 3 | 0.010 | 0.0125 | 6.0 x 10-7 |
What is the overall order of this reaction?
- 0
- 1
- 2
- 3
- 4
2NO2(g) <==> N2O4(g)
has the following experimentally determined rate law:
Rate = k[NO2]2 where k = 400 L mol-1 s-1
How long (in seconds) would it take for a sample of NO2 with an initial concentration of 0.50 M to decrease to a concentration of 0.010 M?
USE THE FOLLOWING INFORMATION TO ANSWER THE NEXT TWO (2) QUESTIONS.
2H2O2(aq) --> 2H2O(l) + O2(g)
The experimentally determined rate law is:
Rate = (0.056 s-1)[H2O2]
- The temperature of the system.
- The geometry or orientation of the collision.
- The velocity of the reactants at the point of collision.
- The concentrations of the reactants.
- All of the above influence the rate.
- A catalyst changes the temperature of a reaction.
- The mechanism of a reaction will change when a catalyst is added.
- A catalyst provides a different activation energy for a reaction.
- A catalyst changes the speed of a reaction, but not the equilibrium constant.
USE THE FOLLOWING INFORMATION TO ANSWER THE NEXT THREE (3) QUESTIONS.
Mnn+
Tl+ + 2Ce4+ -------> Tl3+ + 2Ce3+
The experimentally determined rate law is:
Rate = k[Ce4+][Mn2+]
with the following proposed mechanism involving ions of manganese:
| Step 1: | Ce4+ + Mn2+ --> Ce3+ + Mn3+ |
| Step 2: | Ce4+ + Mn3+ --> Ce3+ + Mn4+ |
| Step 3: | Tl+ + Mn4+ --> Tl3+ + Mn2+ |
- Mn4+
- Mn3+
- Mn2+
- All of the above.
- There is not enough information provided to answer the question.
- Step 1
- Step 2
- Step 3
- All of the above.
- There is not enough information provided to answer the question.
- 0
- 1
- 2
- 3
- 4
I-(aq) + OCl-(aq) --> IO-(aq) + Cl-(aq)
and the following initial concentration and initial rate data for this reaction,
| [I-], M | [OCl-], M | initial rate, M s-1 |
|---|---|---|
| 0.1000 | 0.0500 | 3.05 x 10-4 |
| 0.3000 | 0.0100 | 1.83 x 10-4 |
| 0.2000 | 0.0500 | 6.10 x 10-4 |
| 0.3000 | 0.0200 | 3.66 x 10-4 |
Which of the following is the correct rate law for this reaction?
- Rate = k[I-]
- Rate = k[OCl-]
- Rate = k[I-]2
- Rate = k[I-]2[OCl-]
- Rate = k[I-][OCl-]
- the mechanism of the reaction
- the spontaneity of the reaction
- the rate of the reaction
- the activation energy of the reaction
- all of the above can be affected/changed by a catalyst
- Rate constants can have negative values.
- The order of a reactant appearing in the rate law must always be a positive integer.
- The order of each reactant appearing in the rate law is equal to the stoichiometric coefficient for that reactant in the overall balanced equation.
- Reaction rates can have negative values.
- The rate of disappearance of a reactant is generally not constant over time.
CH3COCH3(aq) + Br2(aq) + H+(aq) CH3COCH2Br(aq)
and the following initial rate data:
| [CH3COCH3], M | [Br2], M | [H+], M | initial rate, M s-1 |
|---|---|---|---|
| 0.30 | 0.050 | 0.050 | 5.70 x 10-5 |
| 0.30 | 0.10 | 0.050 | 5.70 x 10-5 |
| 0.30 | 0.050 | 0.10 | 1.14 x 10-4 |
| 0.60 | 0.050 | 0.20 | 4.56 x 10-4 |
| 0.60 | 0.050 | 0.050 | 1.14 x 10-4 |
Which of the following is the correct rate law for this reaction?
- Rate = k[CH3COCH3][Br2][H+]
- Rate = k[CH3COCH3]2[Br2]2[H+]
- Rate = k[CH3COCH3][Br2]
- Rate = k[CH3COCH3][H+]
- Rate = k[CH3COCH3]
2HI(g) --> H2(g) + I2(g)
| time, sec | [HI], M |
|---|---|
| 0 | 1.000 |
| 100 | 0.899 |
| 200 | 0.806 |
| 300 | 0.735 |
| 400 | 0.676 |
Calculate the concentration (in M) of HI after 1100 seconds.
- rates of chemical reactions
- reaction mechanisms
- factors that influence rates of chemical reactions
- i only
- i and ii
- i and iii
- ii and iii
- i, ii and iii
2NO2(g) --> N2O4(g)
Calculate the value of the rate constant for this reaction if it takes 0.005 seconds for the initial concentration of NO2 to decrease from 0.50 M to 0.25 M.
2NO2(g) --> N2(g) + 2O2(g)
| k, M-1 s-1 | temperature, K |
|---|---|
| 0.522 | 319 |
| 0.755 | 329 |
| 1.70 | 352 |
| 4.02 | 381 |
| 5.03 | 389 |
Calculate the value (in kJ/mol) of the activation energy for this reaction.
- activation energy
- temperature
- reactant concentration
- An increase in any of these will increase the rate.
- Rate will not be affected by any of these.
- decreasing the activation energy
- increasing the concentrations of the reactants
- increasing the temperature
- adding a catalyst
- None of these will decrease the rate.
S2- + 4 Cl2 + 8 OH- --> 8 Cl- + SO42- + 4 H2O
is measured at a particular moment in time and it is
found that -![]() [S2-]/
[S2-]/![]() t = 2.0 x 10-2
mol L-1 s-1. At this same moment in
time, what is the rate (in mol L-1 s-1)
at which Cl- is being formed?
t = 2.0 x 10-2
mol L-1 s-1. At this same moment in
time, what is the rate (in mol L-1 s-1)
at which Cl- is being formed?
BH4-(aq) + 4 H2O(l) --> B(OH)4-(aq) + 4 H2(g)
the following data were obtained:
| time, h | [BH4-], M |
|---|---|
| 0 | 0.100 |
| 24 | 0.088 |
| 48 | 0.077 |
| 72 | 0.068 |
| 96 | 0.060 |
Calculate the value of the rate constant for this reaction if the rate law is: Rate = k[BH4-].
2A --> B + C
where the initial concentration of A is 0.48 M. Assuming that this reaction is second-order in A, calculate how long (in seconds) it would take for the concentration of A to reach 0.24 M if A is initially decomposing (i.e., at t = 0) at a rate of 4.0 x 103 mol L-1 s-1.
- the values of the rate constant and the half-life are both large.
- the values of the rate constant and the half-life are both small.
- the value of the rate constant is small and the value of the half-life is large.
- the value of the rate constant is large and the value of the half-life is small.
- it is not possible to determine the values of the rate constant or the half-life.
2 NO(g) + O2(g) --> 2 NO2(g)
- Rate = k(NO)2
- Rate = k(NO)2(O2)
- Rate = k(NO2)2
- Rate = K(O2)
- Cannot be determined from the information given.
- increases as reactant is consumed.
- is independent of temperature.
- depends on the concentration of the products.
- decreases as reactant is consumed.
- is independent of concentration of reactants or products.
USE THE REACTION AND DATA BELOW TO ANSWER THE NEXT FOUR (4) QUESTIONS.
NO(g) + O3(g) --> NO2(g) + O2(g)
| initial [NO], M | initial [O3], M | initial rate of reaction, M s-1 | |
|---|---|---|---|
| trial 1 | 2.1 x 10-6 | 2.1 x 10-6 | 1.6 x 10-5 |
| trial 2 | 4.2 x 10-6 | 2.1 x 10-6 | 3.2 x 10-5 |
| trial 3 | 6.3 x 10-6 | 2.1 x 10-6 | 4.8 x 10-5 |
| trial 4 | 6.3 x 10-6 | 4.2 x 10-6 | 9.6 x 10-5 |
| trial 5 | 6.3 x 10-6 | 6.3 x 10-6 | 14.4 x 10-5 |
- Zero order in NO and first order in O3.
- First order in NO and first order in O3.
- Second order in NO and zero order in O3.
- Second order in NO and second order in O3.
- Independent of the concentration of O3.
- 6.7 x 10-5
- 7.6
- 3.6 x 106
- 1.8 x 1012
- 8.2 x 1017
- 3.11 x 10-16 mol/L s
- 7.54 x 10-11 mol/L s
- 1.98 x 101 mol/L s
- 9.95 mol/L s
- 3.6 x 10-5 mol/L s
- straight line with a positive shape.
- curve in which the (NO) decreases rapidly at first and then slows down until a minimum concentration is achieved.
- straight line with a negative slope.
- straight line with a slope of zero.
- curve in which the (NO) increases rapidly at first and then slows down until a maximum concentration is achieved.
2 NO2(g) + Cl2(g) --> 2 NO2Cl(g)
N2O5(g) --> 2 NO2(g) + 1/2 O2(g)
How long will it take for the concentration of N2O5 to decrease from 0.050 mol/L to 0.030 mol/L?
- 2.41 minutes
- 3.60 minutes
- 9.63 minutes
- 14.3 minutes
- 19.3 minutes
USE THE REACTION AND DATA BELOW TO ANSWER THE NEXT THREE (3) QUESTIONS.
2 N2O5(g) --> 4 NO2(g) + O2(g)
| [N2O5], M | time, s |
|---|---|
| 5.00 | 0 |
| 3.52 | 500 |
| 2.48 | 1000 |
| 1.75 | 1500 |
| 1.23 | 2000 |
- zero-order in N2O5.
- half-order in N2O5.
- first-order in N2O5.
- second-order in N2O5.
- third-order in N2O5.
- between 0 and 12 seconds.
- between 12 and 20 seconds.
- between 120 and 1,200 seconds.
- between 1,200 and 12,000 seconds.
- between 12,000 and 120,000 seconds.
- between 0.001 and 0.010 mol/L
- between 0.010 and 0.10 mol/L
- between 0.10 and 0.4 mol/L
- between 0.4 and 0.8 mol/L
- between 0.8 and 1.2 mol/L
- Battle of Actium in 31 BC, Octavian defeating Mark Anthony.
- Battle of Lugdunum (Lyon), 197 AD, Septimius Severus defeating Clodius Albinus.
- Battle of Hastings, 1066 AD, William of Normandy defeating Harold II of England.
- Battle of Yorktown, 1781 AD, the Marquis de Lafayette defeating Lord Cornwallis.
- Battle of Waterloo, 1815 AD, the Duke of Wellington defeating Napoleon Bonaparte.
2 NO2(g) + F2(g) --> 2 NO2F(g), rate = k(NO2)(F2)
Which of the following mechanisms provides the best explanation of the experimental rate law?
(a) 2NO2 + F2 --> 2NO2F (one step)
(b) NO2 + F2 --> NO2F + F (fast)
NO2 + F --> NO2F (slow)
(c) F2 --> 2F (slow)
2NO2 + 2F --> 2NO2F (fast)
(d) NO2 + F2 --> NO2F + F (slow)
NO2 + F --> NO2F (fast)
(e) None of these mechanisms are consistent with the experimental data.
NO (g) + O3 (g) -> NO2 (g) + O2 (g)
Consider the following mechanism for this reaction:
| (1) | O3(g) -> O2(g) + O(g) | slow |
| (2) | NO(g) + O(g) -> NO2(g) | fast |
Which one of the following rate laws would be consistent with the mechanism proposed above?
- Rate = k[NO][O][O3]
- Rate = k[NO]
- Rate = k[NO][O3]
- Rate = k[NO][O]
- Rate = k[O3]
- O and NO
- NO and O3
- NO only
- O only
- NO2 only
2 NO (g) + 2 H2 (g) -> N2 (g) + 2 H2O (g)
Consider the following mechanism for this reaction:
| (1) | 2NO(g) <=> N2O2(g) | fast |
| (2) | N2O2(g) + H2(g) -> N2O(g) + H2O(g) | slow |
| (3) | N2O(g) + H2(g) -> N2(g) + H2O(g) | fast |
Which one of the following rate laws would be consistent with the mechanism proposed above?
- Rate = k[NO]2[H2]2
- Rate = k[NO][H2]
- Rate = k[H2]2[N2]
- Rate = k[NO]2[H2]
- Rate = k[NO]2
- zero order
- first order
- second order
- third order
- fourth order
2 N2O5 (g) -> 4 NO2 (g) + O2 (g)
and the following experimental data:
| [N2O5], M | rate, M s-1 |
|---|---|
| 0.100 | 6.96 x 10-4 |
| 0.050 | 3.48 x 10-4 |
| 0.025 | 1.74 x 10-4 |
What is the value of the rate constant, k, for this reaction?
2 H2S(g) + O2(g) --> 2 S(s) + 2 H2O(l)
Which of the following statements is TRUE?
- The reaction is 3rd order overall.
- The rate law is given by rate = k[H2S]2[O2].
- The reaction is 2nd order overall.
- The rate law is given by rate = k[H2S][O2].
- The rate law cannot be determined from the information given.
| time, s | [HO2], molecules cm-3 | ln [HO2] | 1/[HO2] |
|---|---|---|---|
| 0 | 1.0000 x 1011 | 25.3 | 1 x 10-11 |
| 2 | 0.5000 x 1011 | 24.6 | 2 x 10-11 |
| 6 | 0.2500 x 1011 | 23.9 | 4 x 10-11 |
| 14 | 0.1250 x 1011 | 23.2 | 8 x 10-11 |
| 30 | 6.225 x 109 | 22.6 | 1.6 x 10-10 |
These data indicate that the decay of HO2 occurs by a second order process BECAUSE:
- A plot of 1/[HO2] versus time is linear with a slope of positive k.
- The half-life of the reaction is 2 seconds.
- A plot of ln[HO2] versus time is linear with a slope of positive k.
- The rate of the reaction increases with time.
- The plots of 1/[HO2] and ln[HO2] versus time are both linear.
N2O5(g) --> 2 NO2(g) + 1/2 O2(g)
The experimental rate law is -![]() [N2O5]/
[N2O5]/![]() t = k[N2O5].
At a certain temperature the rate constant is k = 5.0 x
10-4/second. In how many seconds will the
concentration of N2O5 decrease to
one-tenth of its initial value?
t = k[N2O5].
At a certain temperature the rate constant is k = 5.0 x
10-4/second. In how many seconds will the
concentration of N2O5 decrease to
one-tenth of its initial value?
- 2.0 x 103 seconds
- 4.6 x 103 seconds
- 2.1 x 102 seconds
- 1.4 x 103 seconds
- 5.0 x 10-3 seconds
NO2(g) + CO(g) --> NO(g) + CO2(g)
Which of the following mechanisms is consistent with these data?
- NO2 + 2 CO <=> N + 2 CO2,
(fast)
N + NO2 --> 2 NO, (slow) - NO2 + 2 CO <=> N + 2 CO, (slow)
N + NO2 --> 2 NO, (fast) - NO2 + NO2 <=> NO3
+ NO, (fast)
NO3 + CO --> NO2 + CO2, (slow) - NO2 + NO2 --> NO3
+ NO, (slow)
NO3 + CO --> NO2 + CO2, (fast)

| #1 | #2 | #3 | #4 | |
|---|---|---|---|---|
| (a) | catalyst | catalyst | activated complex | product |
| (b) | reactants | activated complex | intermediate | product |
| (c) | reactants | activated complex | catalyst | product |
| (d) | reactants | intermediate | activated complex | product |
| (e) | reactants | intermediate | activated complex | catalyst |
- The total energy of the two colliding molecules is less than some minimum amount of energy.
- Molecules cannot react with each other unless a catalyst is present.
- Molecules that are improperly oriented during collision will not react.
- Molecules in different states of matter cannot react with each other.
- 1 and 2
- 1 and 3
- 2 and 3
- 1 and 4
- 3 and 4
- Endothermic reactions.
- Reactions performed at low temperature.
- Equilibrium reactions.
- Non-spontaneous reactions.
- Reactions with large activation energies.
CH3CH2Br + OH- --> CH3CH2OH + Br-
the form of the rate law is:
Rate = k[CH3CH2Br][OH-]
Which of the following are the correct units for the rate constant, k?
- s-1
- mol L-1 s-1
- L mol-1 s-1
- L2 mol-2 s-1
- L3 mol-3 s-1
- increase by a factor of two.
- increase by a factor of four.
- increase by a factor of eight.
- increase by a factor of nine.
- remain the same.
2 MnO4- (aq) + 5 HNO2 (aq) + H+ (aq) --> 2 Mn2+ (aq) + 5 NO3- (aq) + 3 H2O (l)
Calculate the rate (in M s-1) at which the HNO2 concentration is decreasing if the MnO4- is decreasing at a rate of 0.024 M s-1.
2 MnO4- (aq) + 5 H2C2O4 (aq) + 6 H+ (aq) --> 2 Mn2+ (aq) + 10 CO2 (g) + 8 H2O (l)
Consider the following initial concentration and initial rate data for this reaction:
| [MnO4-], M | [H2C2O4], M | [H+], M | initial rate, M s-1 |
|---|---|---|---|
| 0.0010 | 0.0035 | 0.0010 | 7.0 x 10-4 |
| 0.0020 | 0.0035 | 0.0010 | 2.8 x 10-3 |
| 0.0020 | 0.0070 | 0.0010 | 5.6 x 10-3 |
| 0.0020 | 0.0070 | 0.0020 | 5.6 x 10-3 |
Calculate the value of the rate constant, k, for this reaction.
H2 (g) + 2 NO (g) -> N2O (g) + H2O (g)
A study of initial concentrations (ha, ha!) versus initial rate at a certain temperature yields the following data for this reaction (ha, ha!):
| [H2], M | [NO], M | initial rate, M s-1 |
|---|---|---|
| 0.1000 | 0.5000 | 2.560 x 10-6 |
| 0.2000 | 0.3000 | 1.843 x 10-6 |
| 0.1000 | 0.3000 | 9.216 x 10-7 |
| 0.2000 | 0.6000 | 7.373 x 10-6 |
Calculate the value of the rate constant for this reaction (ha, ha!).
- 669oC
- 402oC
- 371oC
- 350oC
- 183oC
2C4H6(g) --> C8H12(g)
and the experimental data shown below,
| [C4H6], M | rate, M s-1 |
|---|---|
| 1.6 x 10-2 | 3.58 x 10-6 |
| 8.0 x 10-3 | 8.96 x 10-7 |
| 4.0 x 10-3 | 2.24 x 10-7 |
| 2.0 x 10-3 | 5.60 x 10-8 |
The order of the reaction with respect to C4H6 is:
- half order.
- first order.
- second order.
- third order.
- fourth order.
- 1 half-life
- 2 half-lives
- 3 half-lives
- 4 half-lives
- 5 half-lives
NO2(g) + CO(g) --> NO(g) + CO2(g)
depends only on the concentration of NO2 below 225oC. At a temperature below 225oC, the following data were obtained.
| time, s | [NO2], M |
|---|---|
| 0 | 1.000 |
| 3.00 x 103 | 0.613 |
| 9.00 x 103 | 0.346 |
| 2.00 x 104 | 0.192 |
| 5.00 x 104 | 0.087 |
| 9.00 x 104 | 0.050 |
Calculate the value of the rate constant for this reaction.