

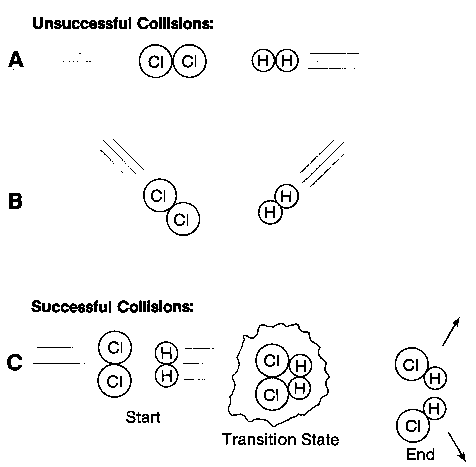
Chemical reactions are processes in which matter changes from one chemical form to another through the rearrangement of atoms. There are many factors affecting the likelihood of a reaction taking place. Some of these are the nature of the reactants, the concentration of the reactants and the temperature of the reaction site. All of these determine the number of "effective collisions" that are taking place. Molecules are always moving and bumping into one another but if they do not bump into each other with the right speed and direction, a reaction will not take place. If the reaction proceeds to completion this usually means that one of the reactants is completely used up before the other, the compound that disappears fist is called the limiting reagent.
Figure 1: ineffective collisions vs effective collisions
Chemical reactions are often described by means of a chemical equation, which specifies the compounds that take part in hte reaction either as reactants or products. The formulae for the reactants are placed on the left side of the equation and the reactants on the right, with an arrow in between to specifiy the direction of the reaction.
ie. 2H2O + O2 -------> H2O
In a chemical reaction, the number of atoms of each element is conserved - the number on the left side equals the number on the right. Therefore a chemical equation that has an equal number of atoms of each element on both sides is said to be a balanced equation. The number appearing in front of the chemical formulae is said to be the stoiciometric coefficient and they indicate equal numbers of atoms of every element on each side of the equation. The physical state of each substance is often included in the equation by means of a symbol placed in brackets: solid, (s); liquid, (l); gas, (g); ions in solution, (aq). Also, in a chemical equation the electric charge of the ions taking part in the reaction is conserved. A chemical equation should always be balanced!!!!
There are several types of reactions that need to be considered in chemistry:
Decomposition is a reaction where a single compound breaks up into two or more simpler compounds or elements. This is often accomplished by heating the compound. If the decomposition products are prevented from escaping, equilibrium forms between the reactant and the products.
ie. CaCO3(s) -----> CaO(s) + CO2(g)
Synthesis reactions are the opposite of decomposition reactions, where two or more elements or compounds form a single compound.
ie. C(s) + O2(g) -----> CO2(g)
If a solid substance separates from solution by combination of ions or molecules present in the solution, that solid is called a precipitate. The ions that do not take part in the precipitation reaction are called spectator ions.
ie. AgNO3(aq) + KCl(aq) -----> AgCl(s) + KNO3(aq)
In an acid-base reaction, an acid reacts with a base to produce a salt and water. The chemistry of an acid-base reaction is essentially the chemistry of hydronium ions.
ie. HCl + NaOH -----> NaCl + H2O
acid + base -----> salt + water
An atom is substituted for an atom in a compound to make a new compound.
ie. CH4(g) + Cl2(g) -----> CH3Cl(g) + HCl(g)
Ususally involves the heating of the reactants and always results in products of carbon dioxide and water.
ie. CH4(g) + O2(g) -----> CO2(g) + O2(g)
These reactions are called redox reactions. The concepts of oxidation and reduction depend on the idea of electron transfer between atoms. The transfer of electrons results in a change in the oxidation numbers of the atoms.
ie. 3Zn(s) + 2OH-(aq) + 2CrO42-(aq) + 8H2O(l) ----> 3Zn(OH)42-(aq) +2Cr(OH)3(s)
There are several steps to balancing a redox reaction:
1. Identify the reactant which is being oxidized and the reactant which is being reduced, by examining the oxidation numbers.
2. Write the "skeleton" half-reactions showing the oxidation and reduction processes and balance them with respect to all elements except hydrogen and oxygen.
3. Balance the oxygen in each half reaction using water.
4. Balance the hydrogen using hydronium ions.
5. Balance electric charge using electrons.
6. Multiply the two half reactions by coefficients such that the oxidation half reaction supplies the same number of electrons as are consumed by the reduction half reaction.
7. Add the two half reactions together to give the complete reaction and tidy the reaction by cancelling any ions or molecules which appear on both sides of the reaction.
 For
more information on chemical reactions, click
here
For
more information on chemical reactions, click
here
 All bolded words have a better
explanation in the definitions section!!!!
All bolded words have a better
explanation in the definitions section!!!!