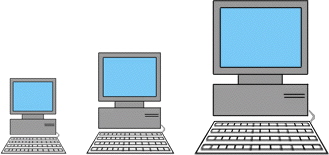
Each electron in an atom is described by four different quantum numbers. Three of these quantum numbers (n, l, and m) represent the three dimensions to space in which an electron could be found. A wave function for an electron gives the probability of finding the electron at various points in space. A wave function for an electron in an atom is called an atomic orbital. The fourth quantum number (ms) refers to a certain magnetic quality called spin.
The n quantam number relates to the size of the atomic orbital. n can have any positive integer value from 1 to 7. The smaller the n, the lower the energy, the higher the value of n, the higher the energy. In the case of any single-electron atom, or hydrogen atom, n is the only quantum number which determines the energy. The size of an orbital depends on n. The larger the orbital, the larger the value of n. Orbitals of the same quantum state belong the the same shell. To use an analogy for n, why not relate it to the size of a computer, where larger values would represent larger houses.

l can have any integer value from 0 to 3. This quantum number distinguishes orbitals of a given n value which have different states. Or, the secondary quantum number gives the shape of the orbital so the analogy can be made to the shape of the computer with larger values associated with computers with more components.
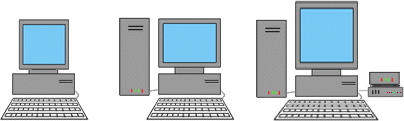
The third quantum number has to do with the orientation of an orbital in a magnetic field. Because of this, we can relate its values to different directions the computer might be facing.

The final quantum number is the spin quantum number, it describes the spin orientation of an electron.
The electron configuration of an atom is the particular distribution of electrons among available shells. It is described by a notation that lists the subshell symbols, one after another. Each symbol has a subscript on the right giving the number of electrons in that subshell. For example, a configuration of the lithium atom (atomic number 3) with two electrons in the 1s subshell and one electron in the 2s subshell is written 1s22s1.
| sublevel | orbital | maximum # of electrons |
| s | 1 | 2 |
| p | 3 | 6 |
| d | 5 | 10 |
| f | 7 | 14 |
The notation for electron configuration gives the number of electrons in each subshell. The number of electrons in an atom of an element is given by the atomic number of that element.
On the left we have a diagram to show how the orbitals of a subshell are occupied by electrons. On the right there is a diagram for the filling order of electrons in a subshell.

Here are some examples that show how to use the filling order diagram to complete the electron configuration for a certain substance.
| Element | # of Electrons in Element | Electron Configuration |
| He | 2 | 1s2 |
| Li | 3 | 1s22s1 |
| Be | 4 | 1s22s2 |
| O | 8 | 1s22s22p4 |
| Cl | 17 | 1s22s22p63s23p5 |
| K | 19 | 1s22s22p63s23p64s1 |
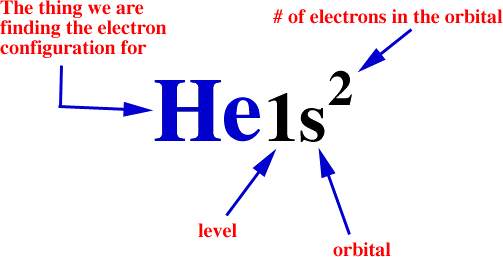
Often times you will be asked to find the electron configuration for something that looks like this:
53I
The 53 denotes the number of electrons in an atom of iodine. You would now proceed to do the electron configuration by looking at the filling order chart.
1s22s22p63s23p64s2
3d104p65s24d105p5
With increasing atomic number, the electron configuration of the atoms display a periodic variation. Because of this the elements show periodic variations of both physical and chemical behavior. The periodic law is a law stating that when the elements are arranged by atomic number, their physical and chemical properties vary periodically. We are going to be looking at three physical properties of an atom: atomic radius, ionization energy, and electron affinity.
The size of the electron cloud increases as the principal quantum number increases. Therefore, as you look down the periodic table, the size of atoms in each group is going to increase. When you look across the periodic table, you see that all the atoms in each group have the same principal quantum number. However, for each element, the positive charge on the nucleus increases by one proton. This means that the outer electron cloud is pulled in a little tighter. One periodic property of atoms is that they tend to decrease in size from left to right across a period of the table. So finally we have a good definition for how the atomic radii increases: the atomic radii increases top to bottom and right to left in the periodic table.
The energy needed to remove the most loosely held electron from an atom is known as ionization energy. Ionization energies are periodic. The ionization energy tends to increase as atomic number increases in any horizontal row or period. In any column or group, there is a gradual decrease in ionization energy as the atomic number increases. Metals typically have a low ionization energy. Nonmetals typically have a high ionization energy.
The attraction of an atom for an electron is called electron affinity. Metals have low electron affinities while nonmetals have high electron affinities. The general trend as you go down a column is a decreasing tendancy to gain electrons. As you go across a row there is also a trend for a greater attraction for electrons.
Electronic Structure of Atoms | Periodicity | Top of Page | Chemistry 20 | Donkistry