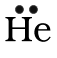
Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an atom's electrons. Dots are drawn around the elements symbol to respresent the electrons in the valence shell of the atom. For example Helium, which has two electrons in its valence shell, would be written as:
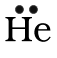
He has two electrons in an s orbital.
![]()
Al has 2 electrons in an s orbial and 1 in a p orbital
The three dots around the symbol stand for the three electrons.
Al has 3 valence electrons (1s22s22p63s23p1). You can determine this by writing out the electron configuration or simply by the Roman numeral III at the top of the family on the periodic table containing Al. The dots are placed on all four sides of the symbol before repeating dots on a side. An example of this is the carbon atom which only has four electrons in its valence shell.

Note: C always makes four bonds; so one electron is promoted from the s orbital to the p orbital and C is represented as this:

Atoms will always promote electrons so they can make more bonds, thus increasing stability.
When the number of electrons in the valence shell is more than four electrons the dots are written as pairs on the side until all the electrons are accounted for. Examples are Fluorine and Sulfur.
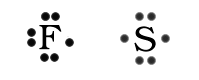
An ionic bond is a chemical bond formed by the electron attraction between positive and negative ions. Ionic bonds are made when an electron from the valence shell of one atom is transfered to the valence shell of another atom. The atom that lost an electron becomes a positive ion and the atom that gains the electron becomes a negative ion.
In NaCl the sodium ion has one less electron than protons so it has a positive charge. The chlorine ion has one more electron than protons so it has a negative charge. Since postives are attracted to negatives the two ions are attracted to each other. The atom that loses an electron becomes a cation which is positive, and the atom that gains an electron becomes an anion which is negative. The nature of the ionic bonds facilitates the formation of ionic solids by attracting other charged atoms to form a solid. The ions are arranged in a crystalline structure with each Na+ ion attracted to several Cl- ions and each Cl- ion attracted to several Na+ ions. There are no NaCl molecules.
Covalent Bonds are chemical bonds formed by the sharing of a pair of electrons between atoms. The nuclei of two different atoms are attracting the same electrons. Therefore, unlike ionic bonds where an electron is moved from one atom to another the electrons are shared.
The Octet Rule is a tendency of atoms in molecules to have eight electrons in their valence shells. (Two for hydrogen atoms.) The octet rule is a general rule, but is not followed by all molecules.
Multiple Bonds are sometimes found in molecules so that the molecules satisfy the octet rule. A single bond (which was discussed earlier) is when a single pair of electrons is shared between the two atoms. A double bond is when two pairs of electrons are shared between two atoms. A triple bond is when three pairs of electrons are shared between two atoms. (Notice a trend?) Double and triple bonds mostly occur when the elements Carbon(C), Nitrogen(N), Oxygen(O) and Sulfur(S) are involved. An example of a molecule with double bonds is Carbon Dioxide (CO2). Notice that each element ends up with eight electrons around it.
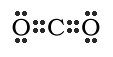
In bonds between atoms of the same element the sharing of the electrons is equal between the two atoms. When two atoms of different elements make a bond, the electrons will not usually be shared equally. The electrons are pulled more toward the more electronegative element. Electronegativity is the measure of teh ability of an atom in a molecule to draw bonding electrons to itself. In general, electronegativity increases from bottom to top and left to right on the periodic table. Fluorine is the most electronegative element since it has a tendency to pick up electrons easily and hold on to them strongly. An element like cesium has a low electronegativity. The unequal sharing of electrons is called a polar covalent bond. The definition of a polar covalent bond is a covalent bond in which the bonding electrons spend more time near one atom than the other.
Now that you understand several types of bonds, a discussion of how to draw the different bonds using Lewis dot notation is in order. There is a generic procedure that can be used to draw almost all molecules. This procedure is as follows:
Total Number of Electrons: 8
![]()
![]()
There are no extra electrons so the drawing is done.
Total Number of electrons : 8
![]()
![]()
Total Number of electrons : 34
7 valence electrons for F and 7 for each Cl. The +1 charge
signifies that one electron has been lost. 7 + 28 - 1 = 34
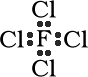

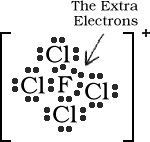
Total Number of electrons : 16
![]()
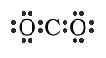
Carbon does not follow the octet rule unless double bonds are formed.
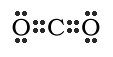
Resonance is another kind of bond that exists
between atoms. In resonance the bond that holds the compound
together is not between two atoms, it is shared between more than
two atoms. Looking at an example is the easiest way to understand
resonace. If we try to draw sulfur dioxide (SO2),
there can be two plausible formulas written. These are: 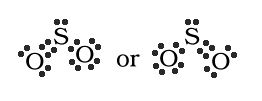
One might conclude that the molecule would be
one or the other but this hypothosis does not fit the
experimental data. During experimentation the bonds were
determined to be the same between sulfur and both of the oxygen
atoms. There were two S-O bonds that were just alike. So neither
of the above formulas are correct. So the proper way to write SO2
is as follows: 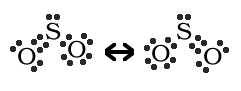
The above notation symbolizes how the two bonds are the same not how the molecule can be one or the other. The actual structure is a composite of these two.
Lewis Dot Notation | Ionic Bonds | Covalent Bonds | Polar Covalent Bonds | Lewis Dot Notation Revisited | Resonance | Top of Page | Chemistry 20 | Donkistry