Oscillating Methanol Explosion
Description: A hot, glowing platinum wire appears to "explode" or poof when placed inside a flask containing a small amount of methanol. After the explosion the wire stops glowing but within about 10 seconds it will start to glow again and then poof....
Concept: Platinum catalyzes the oxidation of methanol. This oxidation occurs on the surface of the platinum wire and causes the wire to glow red. Eventually it will get so hot that their will be a small methanol explosion. The oxygen in the flask is consumed in this explosion, thus stopping the oxidation reaction. When oxygen diffuses back into the flask the oxidation begins again causing the platinum to glow and then an explosion....
Materials:
Safety: The explosion is small and should not be
dangerous, but wear safety goggles and set the shield between the
flask and the students. The flask may get hot, wear gloves to
protect hands. The first explosion my startle you, be careful and
keep the flask steady
Procedure:
Put 10 - 20 mL of Methanol in flask.
Heat the coiled platinum wire for 10 seconds.
Set the wire on the flask so that the platinum coil is
suspended a quarter inch above the methanol in the flask.
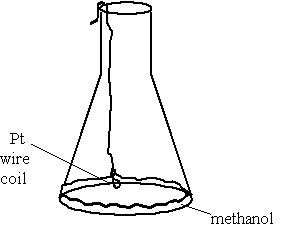
Diagram:

Clean-Up: Set the flask in a fume hood and let the
methanol evaporate.
Notes: The wire was prepared to hang on the edge of the flask such that the platinum coil will be suspended about a quarter inch above the methanol in the flask.
- Keith Dunn developed this demonstration for Ewing's C100 class.
- There is an in-depth article about this demonstration in the
Journal of Chemical Education, vol. 71, no.4, pp 325 -327.