- Length
- Mass
- Time
- Electric Current
- Temperature
- meter
- liter
- cubic centimeter
- milliliter
- degree Celcius
- kelvin
- 10-12
- 106
- 10-9
- 10-2
- 10-3
- 109
- 103
- 10-6
- 10980000000
- 414100
- 0.000095162
- 746.5x107
- 30.84 + 9.74
- 30.84 + 9.74486
- 30.845 + 9.74
- 30.845 + 9.75
- 145 + 1.54x10-6
- 40.79 - 1.18432
- 1.43 x 0.848
- 1.43 x 0.84828
- 136 x 3.0
- (7.601x107) x (8.09x10-4)
- 70.22
- 947731/2
-
a.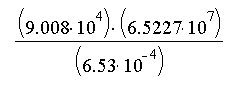
b.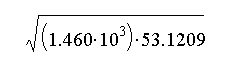
c.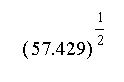
d.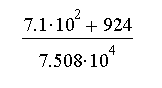
- Convert 78.01 inches into: feet, meters, centimeters, millimeters and kilometers.
- Convert 14511 feet into miles, kilometers and meters.
- Convert 15.42 meters into kilometers, centimeters, millimeters, micrometers, and nanometers.
- Convert 98.6 °F into °C and K.
- Convert 75 miles per hour into: km per hour and m s-1.
- Convert 23.15 m2 into ft2, in2, and cm2
- Convert 500.0 cm3 into in3, m3, L, and mL.