
Molecular Geometry is the general shape of molecules. The shape is governed by how many bonding pairs there are around the central atom, and by how many of those pairs are participating in a bond. Molecular Geometry is an important topic for understanding the properties and reactions of chemicals.
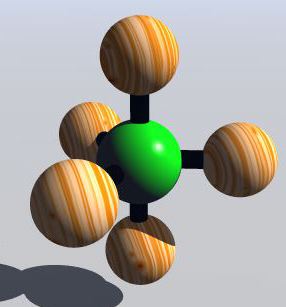
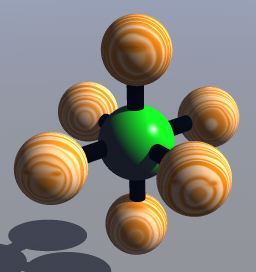
The Valence-shell electron-pair repulsion (VSEPR) Model is a model that states electron pairs in a molecule will be as far apart from one another as they can be because they repel each other. In a molecule that has two valence shell electron pairs the electrons tend to be on opposite sides of the central atom. If there are three electron pairs around the central atom they are in a trigonal planar shape. With four electron pairs the arrangement is called a tetrahedral arrangement. With five electron pairs the arrangement is called trigonal bipyramid. With six electron pairs the arrangement is called an octahedren.
Linear

Trigonal Planar
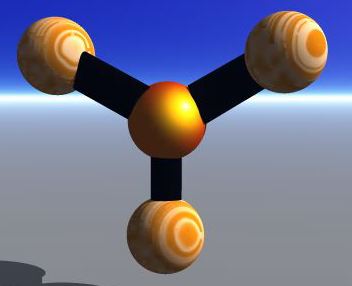
Tetrahedron
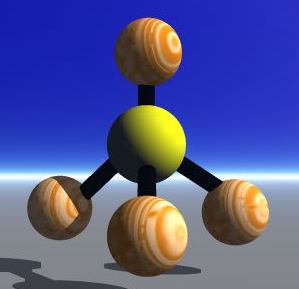
Trigonal Bipyramid
Octahedron
If the electrons are not distributed equally, the molecule is said to be polar. That is, the molecule has a negative end and a positive end. It has two poles and is polar; it has a measurable dipole moment.
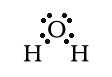
The electrons are not distrbuted evenly so the
water molecule is polar. The negative end of the molecule is the
oxygen end. O is more electronegative than H and pulls the
negative electrons toward itself. Also, there are two lone pairs
around oxygen. 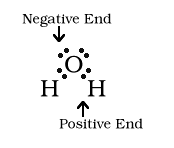
You can write a formula denoting the negative and positive end of the molecule. The sigma (with a plus or minus) above the symbol for the atom is the indicator for which end of the molecule is positive or negative.
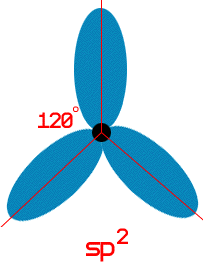
As discussed in the electron configuration section when electrons bond they fill orbitals. One might think that the s, p, and d orbitals are different in the bonds. This would make sense but experimental data shows that all the bonding orbitals in many molecules are the same. The way that this occurs is through Hybridization. The hybrid orbitals are formed by combining s, p and d orbitals. If a molecule has three bonds it needs three orbitals. So the first three orbitals that are used are an s and two p orbitals. The hybrid orbital that is formed is called a sp2 orbital. All three of the orbitals are the same.
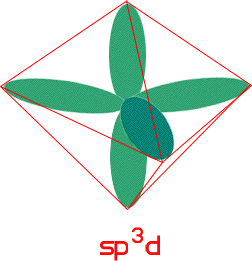
If the molecule has the triginal bipyramid shape then the molecule has sp3d. (This is because it has five bonds so it needs five orbitals.)
Single bonds which are the most prevalent of all bonds are called sigma bonds. These bond orbitals overlap end to end.
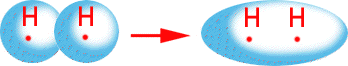
Double bonds which were discussed earlier have one sigma bond and one pi bond. The sigma bond acts the same in a double bond as it does in a single bond. (Overlapping end to end) The pi bond (which is the second bond) is different. The pi bond consists of a sideways overlap of two orbitals.

Triple bonds are just like double bonds except that there are two pi bonds not just one.
VSEPR Model | Polarity | Hybridization | Multiple Bonds | Top of Page | Chemistry 20 | Donkistry