Electrons in Atoms
Atomic Spectra Information
Atomic spectra are useful for identifying different elements. Each element has its own unique atomic spectrum. This section will show how a line spectrum is created and compare it to a continuous spectrum.
A continuous spectrum is one in which the colors blend from one to another. A good example of this is a rainbow, the reds blend into the oranges, which blend into the yellows, then to greens, blues, and violets. There is not a distinct division between the colors in a continuous spectrum. When looking through a diffraction grating at a light bulb, the white light is divided into its component colors - Red, Orange, Yellow, Green, Blue, Indigo, Violet (or ROYGBIV) and you see a rainbow.
![]()
A line spectrum, on the other hand, consists of distinct colors which appear as lines, not as bands of colors. If you looked at a hydrogen discharge tube through a diffraction grating, you would see a distinct red line, green line, and maybe 1-2 violet lines. They do not blend together. The reason for this is illustrated below.
![]()
Electrons are responsible for the lines that are seen. Electrons in an atom are located in different energy levels. When energy is added to the atoms, the electrons which were in the ground state (the lowest energy level possible for the electrons) are raised to an excited state (an energy level above the ground state). The electron has absorbed a quantum of energy to rise to a higher energy level. Remember, a quantum is just a discrete, definite (or exact) amount of energy. Once the electron is in the higher energy level, it cannot maintain this position for long and it falls back to a lower level. As it falls back it must release a quantum of energy. Sometimes this energy is equal to that of a certain color (wavelength) of visible light. The diagrams below illustrate this for hydrogen and show the possible transitions for three of the above colors seen.
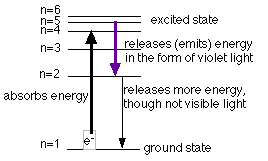
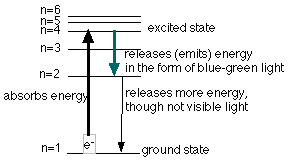
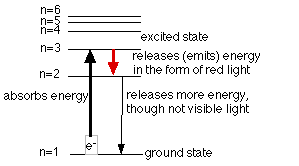
Each element, as mentioned above, has its own fingerprint spectrum. Each element has its own unique energy levels so that different colors are produced.
This is a basic introduction to atomic spectrum. More will be added to this page as time becomes available.