What exactly is matter? Matter is anything that has mass and takes up space. There are two main ways of classifying matter. Matter is classified first by its physical state as a solid, liquid, or gas. Secondly, we classify matter by it chemical constitution as an element, a compound, or a mixture. We are going to learn about the differences between atoms, ions, and molecules, the differences between compounds, elements, and mixtures, and the difference between physical and chemical changes.
To understand what matter is, we first must comprehend the three different states that it can exist in. Those three physical states are solids, liquids, and gases. A good example to illustrate this is water. Water, in its solid state is ice, in its liquid state is liquid water, and in its gaseous state is steam.
Solids usually have a definite shape and a definite volume. However, when a solid is broken into smaller pieces it is not changed chemically. For example if you crush an aspirin into a power it is still a solid just in smaller pieces.
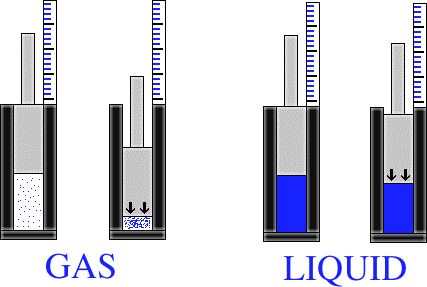
Now we have the problem of distinguishing between a liquid and a gas. What makes a liquid different from a gas is the characteristic of compressibility. A gas is easily compressible, where a liquid is not. Say for instance that you have a piston within an enclosed tube. If the tube is filled with steam, and then the piston is compressed, it is easy to compress the steam with the piston. As a result, the piston travels far into the tube. Now we put water into the enclosed tube. It is not nearly as easy to push the piston down into the tube now. Why? Well, a liquid is a lot harder to compress than a gas. This is because the molecules in the gas are farther apart than the molecules in the liquid.

These two characteristics that we have discussed, how rigid an object is, and an object's ability to be compressed, are used to determine the three basic states of matter
A solid is a form of matter which is made distinct by it rigidity. That is, a solid has a fairly fixed volume and shape, and is harder to compress than a gas or a liquid.
A liquid is a form of matter that is a fairly incompressible. This means that a liqiud basically has a fixed volume, but not a fixed shape. It takes the shape of its container.
A gas is an easily compressible fluid. This means that a given quantity of gas will fit into a container of any size and shape. A gas has neither a definite volume nor a definite shape.
An atom is an extremely small particle of matter that retains its identity during chemical reactions. During the latter nineteenth century a series of experiments showed that atoms are comprised of smaller particles. An atom consists of a nucleus and one or more electrons surrounding the nucleus. The nucleus, the core of the atom, has the majority of the mass of the atom, and a positive charge. An electron is a very light particle which circles the nucleus. It has a negative charge. In an electrically neutral atom, the number of electrons equals the positive charge on the nucleus. The nucleus of the atom is composed of two smaller particles called neutrons and protons. A proton has a positive charge equal in magnitude to the negative charge of an electron. This means that in an electrically neutral atom (one with an equal number of protons and electrons), the postive charge of the protons, combined with the negative charge of the electrons, would result in no charge because they would cancel each other out. A proton's mass, however, is a whopping 1836 times that of the electron. A neutron has a mass almost identical to a proton's, but it has no electrical charge associated with it.
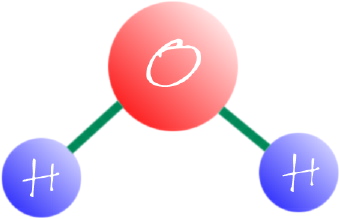
A molecule is a definite group of atoms that are chemically bonded together. They are tightly connected by attractive forces. A molecular formula is a chemical formula that gives the exact number of different types of atoms in a molecule. Some simple molecular substances are carbon dioxide, CO2; ammonia, NH3; and water, H2O. The atoms that are in a molecule are not just stuffed together without any order. The atoms are chemically bonded to one another in order to form a definite arrangement. A structural formula is a chemical formula which shows how the atoms are bonded to one another to form a molecule. A good example is the structural formula for water, H-O-H. Those two horizontal lines connecting the H with the O (hydrogen and oxygen) represent the chemical bonds joining the atoms.
An ion is an electrically charged particle obtained from an atom or chemically bonded group of atoms by adding or removing electrons. Now what this means is that an ion is the result of taking away, or adding, electrons to an atom or a chemically bonded group of atoms. By taking away, or adding, these electrons, the particle takes on an electrical charge. Atoms are electrically neutral as they contain an equal number of positive and negative charges. An atom that adds an extra electron to it becomes a negatively charged ion. This type of ion is called an anion. An atom which loses one or more of its electrons now has a positive charge, and is called a cation. For example, a sodium atom can lose one of its electrons and form a sodium cation. Now, instead of being Na, it would be Na+1. This means that the sodium atom has an overall positive charge of +1. Another example would be a neutral atom of Sulfur, S. If this atom of S were to gain two electrons it would become S-2. The sulfur atom would now have a total negative charge of -2.
It has 16 protons and 18 electrons.
John Dalton (1766-1844) provided us with the Atomic Theory. His theory says that all matter is composed of small particles called atoms.
We now know that atoms are not indivisible , since they
contain protons, neutrons, electrons and other subatomic
particles. However, other than this mistake Dalton's ideas are
essentially correct.
An element is a substance that is composed of only one kind of atom like aluminum, iron, or neon. Today, 109 elements are known and listed on the periodic table.
A compound is a substance of more than one element, chemically combined. A more scientific definition is that a compound is a type of matter composed of atoms of two or more elements chemically combined in fixed proportions. An example of a compound would be water. It is a compound that contains the elements hydrogen and oxygen fixed in the ratio 2 to 1. A compound has new properties unlike the elements which make it up. A compound has a chemical formula such as H2O.
A mixture is a material that can be separated by physical means into two or more substances. A classic example of a mixture lab would be one in which you were presented a mixture of sand, iron filings, and salt. You are told to separate these materials. How do you do that? Well, think about the various physical properties of each material. You use a magnet to separate out the iron filings. You then mix water with the sand and salt mixture. You swish the water, salt and sand around for a while and then filter it. The salt dissolved into the water, so the salt water solution passes through the filter while the sand gets left in the filter. Now we slowly heat up the salt water solution, and evaporate the water, and we are left with salt. In a mixture, the compounds are not in a definite proportion. For example, a teaspoon of salt in a liter of water is salt water, but so is a cup of salt in a liter of water.
A physical change is a change in the form of matter but not in its identity. An example of a physical change would be the dissolving of one thing into another thing. For instance, dissolving sugar into water. The water and the sugar retain their chemical identities and can be separated by physical means. Another example is ice melting to water. Ice and water are both H2O. The identity of the matter is not changed, just the state that it is in.
A chemical change is a change in which one kind of matter is changed into a different type of matter. Some examples of chemical changes: the rusting of your car, setting your shoe on fire, digesting food, and the burning of magnesium metal in oxygen to form magnesium oxide. All of these materials combine chemically with another material , and cannot be separated by any physical means.
Solids/Liquids/Gases | Atoms/Molecules/Ions | Compounds/Elements/Mixtures | Physical & Chemical Changes | Top of Page | Science10 | Donkistry