

In chemistry and physics the idea of the atom is a key concept.
To understand many of the other concepts in chemistry some
knowledge of the atom is necessary. In this section the following
topics will be discussed:
The basic conception of a subject now known as subatomic particle physics dates back to 500 BC when the Greek philosopher Leucippus and his pupil Democritus suggested that matter consists of small, indivisible particles, which they called atoms. For more than 2000 years after this, the notion of atoms lay in obscurity. For quite a long time, people believed that all matter consisted of four elements: earth, fire, air, and water. We now know that atoms do exist, and that some particles smaller than atoms also exist. These subatomic particles are divided into two main groups, the leptons and the hadrons. The best known lepton ("light" particle) is the electron. In order to account for the emission of electrons from the nucleus, the neutrino, an essentially massless neutral particle was postulated. The muon and the tau, both much more massive than the electron, comprise the rest of the lepton family. The hadrons are divided into two groups, the mesons and the baryons. Protons and neutrons are baryons. Mesons and baryons are made of smaller particles called quarks. There are six different quarks: up, down, charmed, strange, top, and bottom. While these are cool names, they convey nothing about the distinct properties of the quark. Each quark comes in three different colors: red blue and green. Again, the color label has nothing to do with the quark's appearance. Baryons are composed of three quarks, mesons are composed of a quark and an antiquark. Now that you have probably been thoroughly confused, move on, and hopefully that confusion will go away.
| Particle | Location | Weight | Charge |
|---|---|---|---|
| Proton | Nucleus | 1.0073 amu | Positive |
| Neutron | Nucleus | 1.0087 amu | Neutral |
| Electrons | Electron Cloud | 0.000549 amu | Negative |
The atomic number of an element is what distinguishes it from all other elements. An atom's atomic number is the number of protons there are in the nucleus. Hydrogen's atomic number is 1. Helium's atomic number is 2. Any atom that has an atomic number of 1 is a hydrogen atom no matter how many electrons or neutrons the atom has.
The mass number is the number of neutrons added to the number of protons. The mass number of the most common isotope can be obtained from the periodic table. If you take the decimal number on the periodic table and round it to the nearest whole number, you have the mass number. For example the atomic weight of Iron(Fe) is 55.847. When rounded it gives a mass number of 56.
The atomic number of Fe is 26. so most Fe atoms
have 30 (56-26) neutrons. In addition, all neutral Fe atoms have
26 protons and 26 electrons. Atoms of the same element with a
different number of neutrons are called isotopes. The most common
isotope of an element is the one that is on the periodic table. 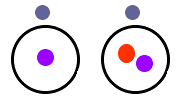

The above graphic shows two isotopes of Hydrogen. The picture
on the left is the most common isotope of hydrogen with one
electron and one proton. The picture on the right is another
isotope of hydrogen with one proton, one electron, and a neutron.
The most common isotope of uranium is uranium-238 which has 92
protons, 92 electrons, and 146 neutrons. Another isotope is
uranium-235 with 92 protons, 92 electrons, and 143 neutrons.
Avogadro's number and the mole are very important to the
understanding of atomic structure. The Mole is like a dozen. You
can have a dozen guitars, a dozen roosters, or a dozen rocks. If
you have 12 of anything then you would have what we call a dozen.
The concept of the mole is just like the concept of a dozen. You
can have a mole of anything. The number associated with a mole is
Avogadro's number. Avogadro's number is 602,000,000,000,000,000,000,000
(6.02 x 1023). A mole of marbles would spread over the
surface of the earth, and produce a layer about 50 miles thick. A
mole of sand, spread over the United States, would produce a
layer 3 inches deep. A mole of dollars could not be spent at the
rate of a billion dollars a day over a trillion years. This shows
you just how big a mole is. This number is so large that it is
usually only represented in scientific notation.
Probably the only thing you will ever have a mole of is atoms or molecules. One mole of magnesium atoms (6.02 x 1023 magnesium atoms) weigh 24.3 grams. 6.02 x 1023 carbon atoms weigh a total of 12.0 grams. 6.02 x 1023 molecules of CO2 gas only weigh a total of 44.0 grams. The decimal number on the periodic table is the atomic mass, the mass of one atom measured in atomic mass units(amu). Amu's are defined to be 1/12 the weight of the most common isotope of Carbon. This number in grams is the mass of 1 mole of that element. For example, 6.02 x 1023 iron atoms weigh only 55.847 grams.(This is equivalent to saying one mole of iron atoms weighs 55.847 grams.) One mole of sulfur weighs 32.066 grams. (This is the same as saying 6.02 x 1023 Sulfur atoms weigh 32.066 grams)
When not measured in grams, the decimal number on the periodic
table is called the atomic mass and is in atomic mass units(amu).
As mentioned earlier, one proton weighs 1.0073 amu and 1 neutron
weighs 1.0087 amu. So the atomic mass is the mass in amus of one
atom of an element, but you rarely use the mass of one atom. Even
if you have a tiny speck of a metal or a microgram of an element,
you have billions and billions of atoms. Thus, the mass in grams
of one mole of an element (the gram atomic weight) is more useful.
SubAtomic Particles | Basic Structure of an Atom | Atomic Number, Mass Number, and Isotopes | Avogadro's Number, The Mole, and Atomic Weight | Top of the page | Science10 | Donkistry