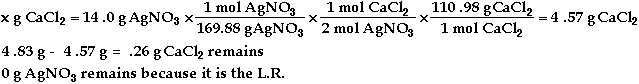a) SR Fe2O3 + 2 Al --> Al2O3 + 2 Fe
b) Combustion C7H16 + 11 O2 --> 7 CO2 + 8 H2O
c) DR BaCl2 + Na2CrO4 --> BaCrO4 + 2 NaCl
d) D 2 HgO --> 2 Hg + O2
e) Combination C600H1000O500 + 50 H2O --> 50 C12H22O11 (large starch molecule converted to sugar)
**f) D 4 C3H5(NO3)3 --> 12 CO2 + 10 H2O + 6 N2 + O2
a) 3 NaBr + H3PO4 --> Na3PO4 + 3 HBr
b) Pb(s) + Mg(NO3)2 (aq) --> No reaction.
c) 2 C8H18 + 25 O2 --> 16 CO2 + 18 H2O
d) 2 KCl --> 2 K + Cl2
e) Mg(s) + 2 AgNO3 (aq) --> 2 Ag(s) + Mg(NO3)2 (aq)
f) F2 (g) + 2 NaCl(aq) --> 2 NaF(aq) + Cl2 (g)
g) 3 Pb(NO3)2 (aq) + 2 FeCl3 (aq) --> 3 PbCl2 (s) + 2 Fe(NO3)3 (aq)
h) 4 Na(s) + O2 (g) --> 2 Na2O (s)
a) Pb+2(aq) + CO3-2(aq) --> PbCO3 (s)
b) 2 Fe (s) + 3 Cu+2(aq) --> 3 Cu (s) + 2 Fe+3(aq)
a)
b) ![]()
a) Mg + 2 HCl --> MgCl2 + H2
b)
![]()
c) ![]()
a)
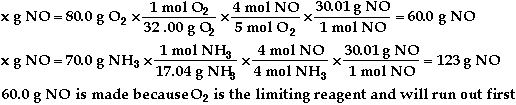
b) ![]()
a)
b) 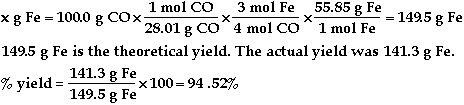
a)
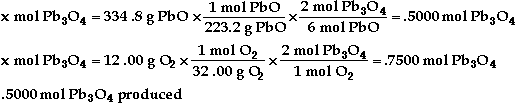
b) ![]()
a)
b) ![]()
pentane
a)
b) ![]()
c) 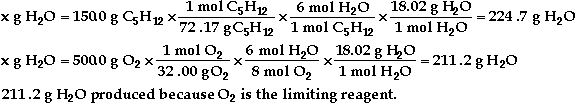
* d) 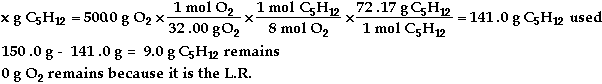
a)
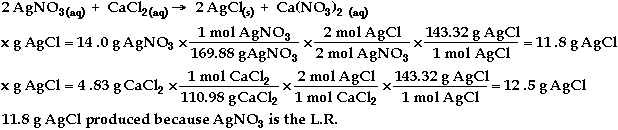
b)