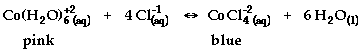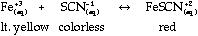Equilibrium Lab and Finding
K and Rate of Reaction Lab
- Equilibrium Lab - Le
Châtelier's Principle
- Introduction/Background: Discuss equilibrium,
reversible reactions, Le Châtelier's principle
and the factors that affect equilibrium, give
examples. The purpose of this lab is to see the
effect of a stress on a reaction.
- Hypothesis: The reaction to be studied is:
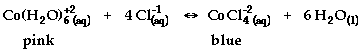
State and explain the effect of each of the
following on the equilibrium of the reaction.
- Adding HCl
- Adding AgNO3 (aq) (Note: Ag+1
reacts with Cl-1 to form AgCl(s))
- Adding H2O
- Procedure:
- Wear goggles at all times.
- Place microplate on white paper.
- Add 5 drops CoCl2 to wells
A1-A10, B1-B10, and C1-C10.
- Add 1 drop HCl to wells A1, B1, and C1.
- Add 2 drops HCl to wells A2, B2, and C2.
- Continue in this manner, increasing the
drops by one each time, until you have
added 10 drops of HCl to A10, B10, and
C10.
- Record observations.
- Part a): Add 1 more drop HCl to each well
in row A. Record observations.
- Part b): Add 5 drops AgNO3 to
each well in row B. Record observations.
- Part c): Add 5 drops H2O to
each well in row C. Record observations.
- Carefully rinse out the microplate in the
sink. Put materials away.
- Data Analysis: Discuss each reaction and its
effects on the equilibrium. Refer back to your
hypothesis. Explain your observations for each
part.
- Evaluation and Conclusion as usual, per the lab format handout.
- Finding Keq
of FeSCN+2
- Introduction/Background:
Discuss Keq and factors that affect
it. Discuss Beer's Law and explain how the
concentration of a solution relates to what you
see (absorbance). The purpose of this lab is to
find the Keq for the formation of
FeSCN+2. This ion is formed by the
reaction:
- Write the Keq
expression.
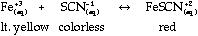
- Procedure:
- Obtain 5 sample tubes,
a light, 2 pipets with a bulb, a sample
of each solution, and 3 beakers.
- Fill one beaker with
distilled water.
- Using a pipet, prepare
five solutions as follows:
- Add 5 mL
0.00200 M SCN-1
solution to each of 5 sample
tubes.
- To tube #1,
add 5 mL of 0.200 M Fe+3
solution. This is your standard
tube.
- Add 10mL 0.200
M Fe+3 solution to a
beaker. Add 15 mL water. Pipet 5
mL of this solution into tube #2.
Set tube #2 aside.
- Pipet 10 mL
from the beaker in step 3 into a
clean beaker. Add 15 mL water.
Pipet 5 mL of this solution into
tube #3. Set tube #3 aside.
- Rinse out the
beaker from step 3 (not the
solution just used for tube #3).
Pipet 10 mL from the beaker in
step 4 into the clean beaker. Add
15 mL water. Pipet 5 mL of this
solution into tube #4. Set aside
tube #4.
- Rinse out the
beaker from step 4 (not the
solution just used for tube #4).
Pipet 10 mL from the beaker in
step 5 into the clean beaker. Add
15 mL water. Pipet 5 mL of this
solution into tube #5. Set aside
tube #5.
- All the tubes
should be of equal heights. Save
the tubes, rinse out the beakers
and the pipets.
- Place the standard
tube (#1) and tube #2 side by side over
the light. Use a small pipet to remove
some solution from the standard
until it appears lighter than tube #2.
Gradually add it back to the standard
until the two tubes appear the same.
- Measure the height of
tube #1 and #2. Record.
- Repeat steps 4 and 5
using the standard (#1) and tube #3, then
tube #4, then tube #5.
- Data Table:
- Calculations: Show all
calculations.
- Calculate the initial
concentrations of both the SCN-1
and Fe+3. For the SCN-1,
use M1V1 = M2V2.
Example: For all tubes: (0.00200 M)(5 mL)
= M2(10 mL total)
M2= 0.00100 M = Initial [SCN-1]
For [Fe+3], use the same
equation. However, you will have two
calculations after tube #1 because the
original solution is diluted in the
beaker, then diluted again when added to
the tube.
- Calculate [FeSCN+2]
for tube #1. Assume the reaction goes to
completion using 0.100 M Fe+3
and 0.00100M SCN-1 and the
reaction given in the introduction.
Calculate the amount of FeSCN+2
that will form.
- Calculate the [FeSCN+2]
for each tube. Use Beer's law, rearranged
to c1l1=c2l2,
where c1 is the concentration
of the standard, l1 is the
depth of the standard, l2 is
the depth of the sample, and c2
is the equilibrium concentration.
- Calculate the Keq
for each tube. Find the equilibrium
concentration of Fe+3 and SCN-1
by using the initial, shift, equilibrium
chart. Once you know the equilibrium
concentrations, you can calculate Keq
using the expression you wrote in the
introduction.
- Data analysis, evaluation, and
conclusion as usual, per the lab format handout.
Questions:
- What assumption was made to find [FeSCN+2]
in the standard test tube?
- Try to find Keq using data
from tube #1. What is the problem?
- What error becomes greater as you
progress to tube #5?
- Rate of
Reaction Lab
- Introduction/Background: The
purpose of this lab is to find how varying the
concentration affects the rate of a reaction.
Include in your background factors that affect
reaction rate, define rate law and order of
reaction. The reaction to be studied in this lab
is
H2O2 + 2 I-1 + 2
H+1 ---> 2 H2O + I2
- Hypothesis: In this lab, the
concentration of H2O2 will
be changed and the rate of production of I2
will be observed. State how [H2O2]
will affect the rate.
- Procedure:
- Obtain two clean, dry,
12 well microstrips.
- In one microstrip, add
4 drops H2O2 to
wells 1-3, add 3 drops H2O2
to wells 4-6, add 2 drops H2O2
to wells 7-9, and add 1 drop H2O2
to wells 10-12.
- In the same microstrip,
add 1 drop water to wells 4-6, add 2
drops water to wells 7-9, and 3 drops
water to wells 10-12.
- In the second
microstrip, to each well add 4 drops
starch solution and 1 drop I-1
solution.
- Carefully invert the
second microstrip over the first. Shake
firmly once to mix. Begin timing. As soon
as a color starts to change in a well,
record the elapsed time for that well.
Continue timing until all the wells have
changed.
- Rinse out the strips in
the sink and shake dry.
- Repeat steps 2-6 for a
second trial.
- For each trial, average
the time in wells 1-3, then wells 4-6,
7-9, and 10-12.
- Data: Set up a chart for two
trials, though you may end up doing more,
depending on what your data looks like.
- Data Analysis: Graph average
time (y-axis) vs. # of drops of H2O2
(x-axis) for each of your trials. You can put
them on one set of axes. Do the graphs make
sense? Discuss the results in terms of how
concentration of H2O2
affects the rate.
- Evaluation and Conclusion as
usual, per the lab
format handout. One question: How would you
find out if [I-1] affected the rate?
Describe the experiment and what results you
would see if the rate was zero order for I-1.
Return to the top of this page.
Return to Chemistry 30